INTENDED USE
The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid chromatographic immunoassay for the qualitative detection of IgG and IgM antibodies to SARS-CoV-2 in human whole blood, serum, or plasma as an aid in the diagnosis of primary and secondary SARS-CoV-2 infections.
PRINCIPLE
The COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is a qualitative membrane-based immunoassay for the detection of SARS-CoV-2 antibodies in whole blood, serum, or plasma. This test consists of two components, an IgG component and an IgM component. In the IgG component, anti-human IgG is coated in IgG test line region. During testing, the specimen reacts with SARS-CoV-2 antigen-coated particles in the test cassette. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with the anti-human IgG in IgG test line region. If the specimen contains IgG antibodies to SARS-CoV-2, a colored line will appear in IgG test line region. In the IgM component, anti-human IgM is coated in IgM test line region. During testing, the specimen reacts with anti-human IgM. IgM antibodies to SARS-CoV-2, if present in the specimen, reacts with the anti-human IgM and the SARS-CoV-2 antigen-coated particles in the test cassette, and this complex is captured by the anti-human IgM, forming a colored line in IgM test line region.
Therefore, if the specimen contains IgG antibodies to SARS-CoV-2, a colored line will appear in IgG test line region. If the specimen contains IgM antibodies to SARS-CoV-2, a colored line will appear in IgM test line region. If the specimen does not contain antibodies to SARS-CoV-2, no colored line will appear in either of the test line regions, indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
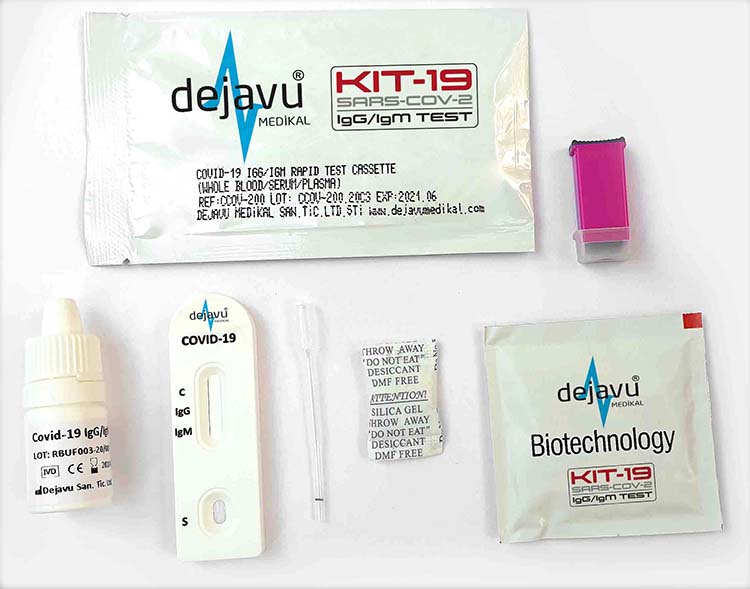
PRODUCT CONTENTS
The test cassette contains to specific antigen conjugated gold colloid particles and anti-human IgM, anti-human IgG coated on the membrane.
Quick Result
Rapid Diagnostic Kit 5-10 min. it gives quick results in it.
%98,89 Precision Ratio
The test result will be 98,89% accurate.
Approved by the Ministry of Health
T.R. It is a sales kit approved by the Ministry of Health.